 [an error occurred while processing this directive]
[an error occurred while processing this directive]
Key Words: arrhythmia, monomorphic, polymorphic, ion channel, discontinuous, propagation, vulnerability, electrogram, torsade de pointes, cardiac, electrophysiology
Running Title: Starmer, et. al. Monomorphic and Polymorphic Reentry
The research was supported in part by CRDF RB1-166 from the Civilian Research and Development Foundation.
Address correspondence:
C. Frank Starmer
Medical University of South Carolina
629C Strom Thurmond Bldg
Charleston, S.C.29425 USA
e-mail: starmerf@musc.edu
phone: (803) 953-6681
fax: (803) 953-6655
Background: Polymorphic ventricular tachycardia often leads to ventricular fibrillation and sudden cardiac death. To explore sources of variability in QRS morphology, we studied reentry in isolated right ventricular tissue from the hibernating suslik (Russian ground squirrel) during episodes of monomorphic VT, polymorphic VT and during spontaneous conversion from polyVT to monoVT.
Methods and Results: In 23 superfused right ventricular preparations, simultaneous endocardial and epicardial isochronal maps were obtained from two 32 element unipolar arrays in contact with epi- and endocardial surfaces. During reentry we measured the reentrant cycle length, the transmural conduction delay, the difference between the first and last activation for each array (activation envelop), and the interval of time between the last activation event associated with cycle n and the earliest activation of cycle n+1 (wave overlap).
Polymorphic reentry was induced by single premature stimuli with a mean s1s2 delay of 80.4 ± 13.1 ms while the s1s2 delay for initiating monoVT was 110.8 ± 16.8 ms (p < .01). In preparations displaying both polyVT and monoVT, the polyVT reexcitation rate was always greater than the monoVT reexcitation rate (p < .01). In preparations where polyVT spontaneously converted to monoVT, wave overlap shifted from + (overlapping waves) to - (no overlap between successive waves), variability in activation parameters diminished and transmural and epicardial conduction paradoxically slowed after conversion to the lower rate monoVT (p < .01). Spontaneous conversion of monoVT to polyVT was never observed. All measures of conduction were significantly more variable during polyVT than during monoVT with variance ratios ranging from 6.8 to 70 (p < .01). Analysis of electrograms during an episode of torsade de pointes revealed transmural Wenkebach conduction that correlated with the torsade cycle.
Conclusions: All activation parameters displayed greater variability during reentrant polyVT than during monoVT. Alterations of QRS morphology during polymorphic reentry was always characterized by large variations in overlap of successive waves. Successive activation waves did not overlap during monoVT. In some episodes of polyVT, the torsade de pointes pattern in QRS morphologies was associated with Wenkebach transmural conduction. These results provide new insights into the complex nature of reentrant mono- and polyVT.
Introduction
Polymorphic ventricular tachycardia has been defined as a high-rate ventricular flutter with temporal changes in the morphology of electrocardiographic QRS complexes (1). PolyVT often leads to ventricular fibrillation and sudden death. Several different models have been explored in order to understand the mechanisms underlying polyVT (2-11). Some studies found evidence of focal activity (3,7) while others found evidence of reentrant activation as the underlying mechanism of the tachycardia (4,5,7,8-11). Recent numerical and in vitro studies (5,8,9,11) demonstrated reentrant polyVT where alterations in the beat to beat QRS morphology was due to instability in the location and/or morphology of the functional unexcited core. The precise nature of polyVT and the determinants of QRS morphology during polyVT, however, remains uncertain.
Each successful excitation of the heart initiates a that propagates away from the region of excitation. The propagating front results from the activation of membrane ionic channels that permit an ionic current to flow in the region of the path of propagation. This current creates an electric field that can be monitored by electrodes either in close proximity to the cardiac surfaces (electrogram, EG) or at distant locations (electrocardiogram, ECG). Repetitive excitation, whether from sinus impulses, focal activation or reentry, will produce similar time-dependent changes in the EG or ECG potentials for each activation cycle, i.e. monomorphic complexes.
During high rate ventricular activation, it is possible that a front arising from sinus, focal or reentrant activation will continue to propagate after the next activation. Specifically, when the time required for a wave to propagate exceeds the reexcitation interval, then there is overlap between these two waves. Because overlapping waves arise from activation currents flowing in two different regions of the heart, there is the possibility that EG / ECG morphology will be significantly altered. Specifically, small variations in activation sequence of a single wave will produce negligible alterations in the EG / ECG morphology. On the other hand, small variations in the activation sequence when two or more wave fragments coexist can make large changes in EG / ECG morphology simply because there are two activive regions of myocardium contributing to the EG/ECG.
To explore the links between QRS morphology and myocardial activation patterns, we studied reentrant arrhythmias in the hibernating suslik (ground squirrel). During hibernation, the suslik heart exhibits an unusually slow heart rate of 1-2/min and short action potentials (14) which facilitates induction of reentrant arrhythmias. The suslik right ventricle is thin (1 - 1.5 mm). With superfusion, electrograms, refractory periods and the threshold of excitation remain stable over a 5-6 hour period. In addition, the surface electrograms have peak-to-peak amplitudes > 1.5 mV which provide a good signal to noise ratio for signal processing.
With two arrays of unipolar electrodes which covered the majority of the epicardial and endocardial surfaces, we monitored surface electrograms during reentry induced by premature stimulation. From these electrograms we constructed isochronal maps and explored the relationship between the activation sequences, the reexcitation intervals and QRS morphology observed in a computed ECG.
We observed that both polymorphic VT and monomorphic VT were easily initiated with programmed stimulation using single premature stimuli. The rate and variability of polyVT was always greater than the rate and variability of monoVT in preparations exhibiting both types of reentry. PolyVT was always characterized by large variations in overlapping waves that produced marked variations in QRS morphology. Transitions from high rate polyVT where wave overlap was positive and quite variable to slower monoVT where wave overlap was negative and showed only small variations were always accompanied by slowing of transmural and epicardial conduction. This observation is consistent with changes in microscopic conduction pathways due to discontinuous propagation. In an episode where the computed-ECG displayed the Torsade de Pointes pattern of undulating peak-to-peak amplitudes, the cycle of QRS morphology changes was associated with Wenkebach cycles of transmural conduction.
Experimental Methods
Tissue Studies
Suslik (Citellus undulantus, 450 - 700 grams) trapped at the end of the summer in Siberia were maintained in near-natural conditions at the Institute of Cell Biophysics in Pushchino Russia. After anesthetization, the heart was rapidly excised and placed in room temperature Tyrodes?s solution of the following composition (mM) NaCl 137, KCl 4, NaHCO3 11.2, NaH2PO4 1.8, MgCl2 1.0, CaCl2 2.0, glucose, 11 The solution was oxygenated with a mixture of 95% O2 +5% CO2 and the pH adjusted to 7.35. Following removal of the heart, the free wall of the right ventricle (approximately 1.5 mm thick) was removed by carefully cutting along the interventricular septum and atrial floor. The size (1.3 cm x 1.3 cm) of the preparation was carefully matched to the size of the electrode array, thus reducing the likelihood of significant electrical activity outside the electrode array. All studies were conducted at 32 +/- 0.5 Co, a temperature where we have observed in the majority of preparations that reentry was readily induced by premature stimulation.
The epicardial surface of the preparation was placed in a 20 ml study chamber on top of a rigid 32 electrode (0.5 mm silver wires) 1.0 cm x 1.0 cm array (lower array). The interelectrode spacing was 2 mm. Each electrode in the lower array was insulated resulting in a total diameter of 1.4 mm. The larger size of the lower array electrodes supported the preparation 1 cm above the floor of the chamber so that the epicardial (lower) surface was adequately perfused. To minimize motion, the extremities of the preparation were gently pinned to an elevated support structure that surrounded the lower array. The 32 electrode upper array ( 0.2 mm tungsten wires with the lower 1 cm insulated) was rotated into alignment with the lower electrode array and then the entire array was gently lowered onto the exposed endocardial surface. The distance between the upper array electrode tips and its supporting structure was 4 cm which provided some flexibility and so that superfusion (12 ml/min) of the endocardial surface would not be inhibited. The stimulating electrode was a coaxial electrode in the upper array with an internal diameter of 0.2 mm and an external diameter of 1.7 mm. The stimulating electrode was usually near the apical portion of the tissue.
The tissue was stimulated at 1 Hz (2x diastolic threshold) for 1 hour in order to permit adaptation with pulses 2-4 msec in duration. During this time, spontaneously occurring arrhythmias often occurred, but with rest for approximately 1 hour, the spontaneous arrhythmias disappeared. The vulnerable period was determined by scanning the diastolic interval with premature stimuli (s2 amplitude = 2x or 4x diastolic threshold) following a 0.5 or 2 Hz train of 10 pulses (s1 amplitude = 2x diastolic threshold) . Data recording started at the beginning of the conditioning pulse train. If there was no response to the s2 test stimulus, the next pulse train was initiated after a period varying from 300 to 500 msec. If there was a response to the test stimulus, basic stimulation was stopped during the time of data sampling. The next sequence of basic and test stimuli were not initiated earlier than 4 seconds following cessation of the previous arrhythmia. The 64 unipolar electrograms were sampled at a 1 kHz rate (12 bits/sample) for periods up to 10 seconds using a Data Translation DT2821G A/D converter connected to a 66 MHz 486 PC and stored for later analysis.
Histologic Studies
Tissue from 4 suslik hearts were used for histologic studies. Tissue was evaluated at 40 min, 2, 4 and 6 hours after the start of 1 Hz stimulation. All conditions were the same as a typical electrophysiologic study. At each time of evaluation, the perfusion system was switched from Tyrodes to Tyrodes + 20 mM Procion (Reactive) Yellow 2 (Sigma) and perfusion continued for 7 minutes. At this time, a small sample of tissue (10 mm x 3 mm) was carefully cut and immediately frozen in liquid N2. The sample was quickly transferred to a cryostat. From the tissue block, 15 micron sections were cut for histologic examination. These sections were placed on glass slides and reviewed using a luminescent microscope (Fluoval, Karl Zeiss) or a Russian spectral microscope equipped with a 250 watt mercury lamp (DRSH-250) with an excitation wavelength, lex = 365 nm. and a band-elimination filter (G243). In parallel with the preparation of sections for microscopic examination, the superfusate solution was switched to standard, dye free Tyrodes and superfusion continued until the next time of evaluation.
Data Analysis
To construct isochronal maps, activation times from each of the 64 unipolar electrograms were first estimated from the maximum negative dV/dt and then manually verified to insure the accuracy of the computer generated estimates. Because of the possibility of multiple waves falling within the same temporal bracket, all electrograms were viewed simultaneously in order to identify the activation events that defined each wave (15).
In addition to maps, three ECGs were computed: an endocardial ECG, a epicardial ECG and a composite ECG (ECGdiff) representing a limb lead ECG. Since the ECG is derived from the potential distribution over the whole surface of the heart, we computed the equivalent of a limb-lead ECG from the differences in the endo- and epicardial ECGs. The ECGdiff was defined by
ECGdiff = ECGendo - K*ECGepi where K is a scale factor for normalizing the amplitude of the epicardial electrograms. This normalization factor was computed from the ratio of potentials measured during test pulses in the absence of tissue (but with the electrode tips within < 1mm of each other and bathed in the perfusate).
Each map was characterized by two measurements:
1)
time of first activation of the endo- and epicardial map, n: Fendo(n),
Fepi(n
2) time of last activation of the endo- and epicardial
map, n: Lendo(n), Lepi(n)
From each pair of epicardial and endocardial maps, we computed four parameters:
1)
reexcitation interval (the interval between successive reentrant cycles:
RIendo(n) = Fendo(n) - Fendo(n-1)
2) activation envelope (interval between the latest and
earliest activation during a single cycle): AEendo(n) = Lendo(n) - Fendo(n)
3) wave overlap: (interval between the latest activation
of cycle, n-1, and the earliest activation of cycle n, illustrated in Figure
1):
WOendo(n) = Lendo(n-1) - Fendo(n)
4) transmural delay (interval between the earliest epicardial
and endocaridal activation for cycle n: TD(n) = Fepi(n) - Fendo(n)
Similar measurements were made from the epicardial maps. Each of these parameters was plotted as a function of time along with the appropriate computed planar and computed-ECGs. Means, standard deviations and coefficients of variation (sd / mean) were computed for each polyVT and each monoVT episode. Comparison of measurements between polyVT and monoVT as well as comparisons of endocardial and epicardial parameters were evaluated using an analysis of variance.
Results
Preparation Viability
Procion yellow dye (reactive yellow 2) was used to assess the viability of the preparation and is not absorbed by viable cells. Microscopic examination resulting in no observed luminescent regions indicated the absence of injury to the tissue. In all studies without induced ischemia, the results showed no significant cellular injury. No luminescence was seen along epicardial or endocardial surfaces. The primary locations of dye uptake appeared to be within the interstitial spaces.
To evaluate the temporal rundown with respect to electrical properties, we compared electrograms and the computed-ECG measured at hourly intervals. We found minimal changes in peak-to-peak amplitude and morphology during this time interval.
Initiation of Reentry
With programmed stimulation, we were able to initiate reentry during
studies lasting as long as 5 hours. In 23 of 28 suslik preparations, we
initiated more than 500 episodes of reentry using premature stimulation.
Figure 2 illustrates typical responses observed.
Only monomorphic reentry was inducible in 6 of 23 preparations. Both monomorphic and polymorphic reentry were initiated by premature stimulation in 17 or 23 preparations. From the 17 preparations displaying polymorphic episodes, 5 exhibited the undulating pattern seen in Torsade de Pointes. The mean s1-s2 interval for initiating monoVT was 110.8 +/- 16.8 ms (n = 30). The mean s1-s2 interval for initiating polyVT was 80.4 +/- 13.05 ms (n = 45) and significantly less than that needed to induce monoVT (p < .01).
For all preparations, after the first second following initiation, the monomorphic reentry rate ranged from 4.9 to 6.5 beats/sec. The quasimonomorphic reentry rate ranged from 5.7 to 11.3 beats/sec while the polymorphic reexcitation rate ranged from 7.9 to 12.9 beats/sec. In the same preparation using this classification scheme, the polymorphic reexcitation rate was always greater than the monomorphic reexcitation rate.
Stability of Reentry
Reentry after initiation by premature stimulation was either stable,
characterized by small variations in reactivation cycle length, wave overlap
and electrogram morphology or unstable, characterized by large variations
in reactivation cycle length, wave overlap and electrogram morphology.
Figure 3 shows the relationship between wave overlap, its variability and
the average reentrant cycle length for the 4 examples described below.
Shown here is an example of continuous polyVT (P), continuous monoVT(M)
and two examples of spontaneous transition from polyVT to monoVT. In all
cases, the coefficient of variation (ratio of standard deviation to mean)
was significantly larger during polyVT than monoVT (p < .01).
Monomorphic Reentry
We performed a detailed analysis of 5 episodes of monoVT, each with
a duration of > 3 seconds. Monomorphic VT was always characterized by small
cycle to cycle variations in the reexcitation interval, activation envelope,
transmural delay and wave overlap. Figure 4 displays the time course of
each measurement which are stable and display very little variability (see
table 1). In this example reentry was initiated by a test stimulus applied
140 ms after the last basic stimulus. The average reexcitation interval
from the endocardial map was 211 ms while the epicardial reexcitation interval
was 212 ms and the reexcitation interval showed very little variability
(sd = 5.0 and 4.7 ms respectively). The synchrony of the endo- and epicardial
reexcitation interval suggested a transmural path which was confirmed from
the maps. The variability in the reactivation interval in all episodes
of monomorphic reentry was less than 3% (n = 5) of the average reactivation
interval, and in this example, the coefficient of variation (std. dev.
/ mean) was .024 (2.4%) for the endocardial surface and .023 (2.3%) for
the epicardial surface.
Endocardial activation propagated more rapidly than epicardial activation. In this episode, the endocardial propagation was complete 22.8 ms after initial activation while epicardial propagation continue an average of 96.6 ms after initial activation, significantly different from the endocardial propagation time, p < .01. Because the endo- and epicardial propagation times were less than the reexcitation intervals (211 and 212 ms), there was no overlap in successive waves. The average endocardial to epicardial transmural delay was 27.8 +/- 0.6 ms and significantly shorter (p < .01) than the epicardial to endocardial delay of 87.9 +/- 2.6 ms (approximated by assuming that the last epicardial activation was coupled to the first endocardial activation of the next reentrant cycle). The sum of the epicardial, endocardial and two transmural paths was 235.1 ms, which is 24 ms greater than the mean reexcitation interval, suggesting that the epicardial to endocardial circuit was activated prior to the last moment of epicardial activation.
The likelihood of multiple waves contributing to the shape of the QRS complex was quite small as indicated by an average wave overlap (time between the last activation of map n and the first activation of map n+1) of -188.1 msec for the endocardial maps and -115.5 msec for the epicardial maps. The QRS morphology was constant and the absence of significant change is consistent with the small variation in all measured conduction parameters.
Polymorphic Reentry
We performed a detailed analysis of 5 episodes of polyVT. Each episode
was > 1.5 seconds in duration. In contrast to monomorphic VT, episodes
of polymorphic VT displayed significant variability in all measured parameters.
Electrograms and computed ECGs for the initial 4 seconds of this episode
are shown in figure 5 and the aveage values of propagation parameters are
shown in table 2. In this example, reentry was initiated by a test stimulus
applied 140 ms after the last basic stimulus. Following the test stimulus,
a reentrant core formed on the endocardial surface 2 mm from the test stimulation
site. Epicardial activation was initiated with breakthrough at the epicardial
site directly opposite the endocardial core location. Over the next few
reentrant cycles, the core drifted toward the center of the endocardial
surface. For the remainder of this episode, the core moved away from the
center and about along 2mm x 6mm region oriented diagonally in the upper
left corner of the electrode array.
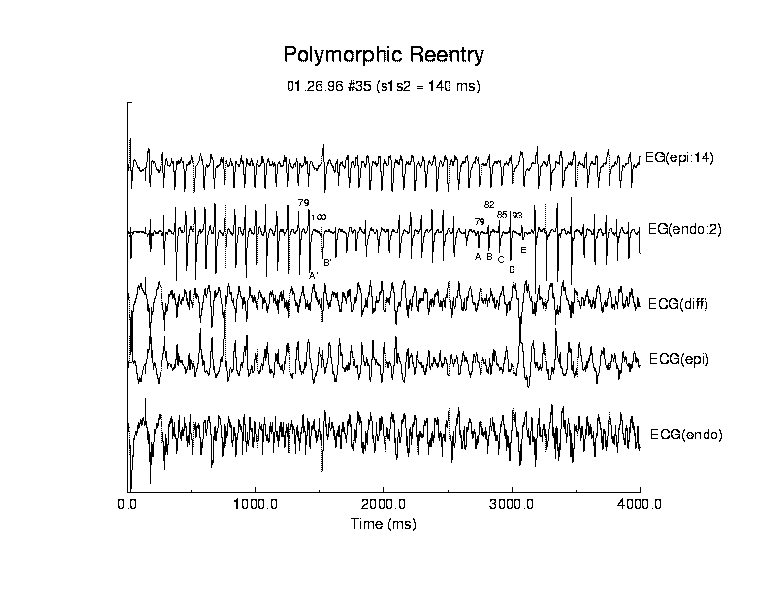
Compared with monomorphic reentry, propagation parameters associated with polyVT revealed significant quantitative differences. PolyVT reexcitation intervals were shorter than monomorphic reexcitation intervals and in this case was 93.3 ms which was significantly shorter than that observed during continuous monomorphic reentry. In addition, the variability of the reexcitation interval was greater (33.0 ms). This is readily seen in the ratio of variability (standard deviation) to the mean reexcitation interval (coefficient of variation) which was 0.35 (endocardial) and 0.37 (epicardial), approximately 10 fold larger than that observed during episodes of monomorphic reentry. In all episodes (n = 5) polymorphic VT displayed reexcitation variability > 10%. Similarly, the activation envelope and transmural delay displayed greater variability during polyVT than during monoVT and in this example, the variability was > 25% of the mean value.
In all examples of polyVT, the activation envelope, i.e. the interval between the first and last activation associated with a reentrant cycle, was comparable to the reexcitation interval. When the activation envelope was greater than the reexcitation interval, then 2 waves overlapped. If either parameter was unstable, then the changing relationship between the 2 overlapping waves altered the morphology of electrogram and computed ECG complexes as seen in figure 5. For comparison, note that during monoVT (table 1) the activation envelop is always less than the reentry interval and there is no overlap of activation waves. On the other hand, note that during the polyVT episode displayed in figure 4, the activation envelope is comparable to the reexcitation interval and both display > 25% variability (table 2). The influence of variable wave overlap is immediately obvious in the computed endocardial and epicardial ECGs. We observed that the wave overlap maxima are often associated with minima in the peak-to-peak ECG amplitude.
In this example, sometimes the electrogram activity at selected sites displayed an inverse relationship between peak-to-peak amplitude of the activation complex and the recovery interval, i.e. a short interval was associated with a large amplitude activation complex while a longer interval was associated with a smaller amplitude complex (see endocardial lead 2, figure 5). In a continuous medium, longer recovery intervals always result in greater recovery of membrane ion channel properties such that the next activation results in a more rapidly propagating front and a larger peak-to-peak amplitude activation complex. Two counter examples are shown in figure 4: example 1; A?B? and example 2; ABCDE. In the first example, the interval preceding the large amplitude A? complex was 79 ms while that preceding the smaller amplitude B? complex was 100 ms. In the second example, interval(AB) = 81 ms, interval(BC) = 82 ms, interval (CD) = 85 ms and interval (DE) = 93 ms. The peak amplitudes increase for each increasing interval until the D-E interval where the peak-to-peak amplitude of E is less that that of any of the preceding complexes (A-D). Such paradoxical results arise from changes in the direction of local propagation at the electrode site, reflecting the underlying structural discontinuities (gap junction cellular connections) that dictate propagation paths.
Transition from Polymorphic to Monomorphic Reentry
Episodes of spontaneous transition from high frequency polyVT to a lower
frequency monoVT provided a basis for comparing both arrhythmias under
nearly equivalent conditions. While we often observed spontaneous transitions
from polyVT to monoVT, we never observed the reverse transition.
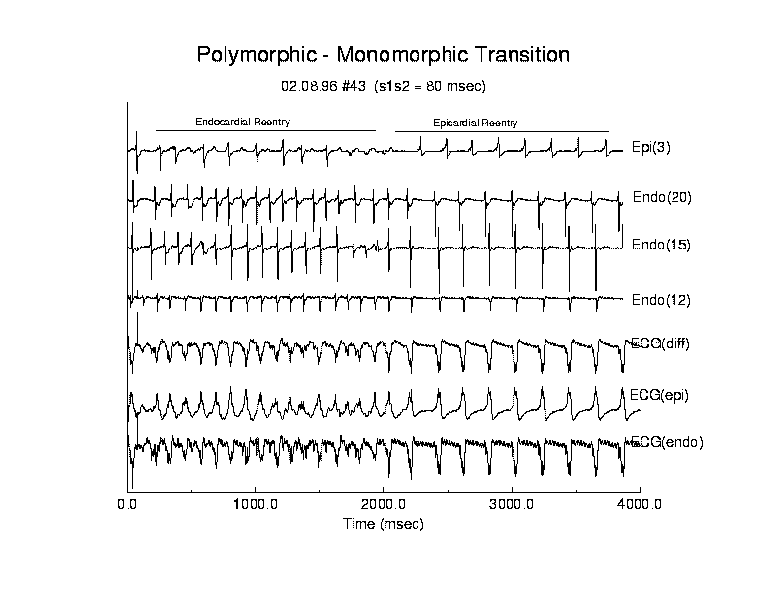
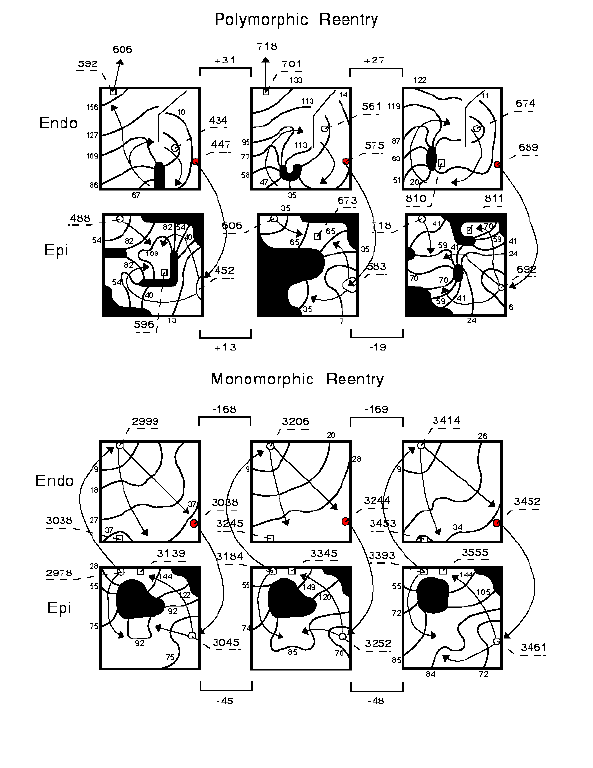
Figure 6 (table 3) illustrates a typical example of spontaneous conversion from polyVT to monoVT. Following test stimulation, a reentrant circuit was evident from the endocardial maps. With each successive reentrant circuit, the core drifted, altering the direction of propagation as seen in the electrogram complexes observed at endocardial site 15. Moreover, during the entire polyVT episode, there was either 2:1 block between endocardial and epicardial activation or alternating fast and slow conduction of epicardial activation as indicated by the alternating high and low amplitude complexes seen in epicardial electrode 3. Note the correlation between peaks of the endo(20) electrogram and the high and low amplitude complexes seen in epi(3).
After 2 seconds of polyVT, transfer of endocardial activation to the epicardial surface was blocked and followed by formation of an epicardial reentrant circuit with the core. During the transition to monoVT, the reexcitation interval almost doubled from 124 ms to 207 ms (endocardial surface) and from 122 ms to 208 ms (epicardial surface) (p < .01) and the variability of the reexcitation interval was simultaneously reduced from 31.7 to 1.9 ms (endocardial) and 25.0 to 2.5 ms (epicardial) (p < .01). In terms of percent changes, the variability in reexcitation interval was reduced from approximately 25% to 1% following spontaneous conversion to a monomorphic rhythm.
The association of wave overlap with the polymorphic nature of the ECG
is readily apparent in this example (figure 7). During the polyVT phase,
the average wave overlap was -28.1 ms and -41.0 ms for the endocardial
and epicardial surfaces. The duration of a typical activation complex (ACD)
was 24 ms. Thus when the overlap was within 2 ACD (48 ms), variation in
wave overlap altered QRS morphology. During the monoVT phase, on the other
hand, the average wave overlap was -174 and -134 ms for the endocardial
and epicardial surfaces and considerably beyond the range of overlap where
QRS morphology can be altered by variations in wave overlap. Note in the
epicardial computed computed-ECG (figure 6), during the polyVT phase, a
reduction in QRS amplitude occurred when the wave overlap was maximum (just
after 1 sec).
Wave overlap was associated with changes in the QRS morphology. Note in figure 6, the small amplitude QRS in the computed ECGdiff complexes at 0.5 sec associated with a local maximum of the wave overlap (panel B). Similarly, during the more stable interval from 1 sec to 1.6 sec, the activation overlap was more negative (less overlap) and relatively stable, consistent with stable morphology of consecutive QRS complexes.
Wenkebach and Torsade de Pointes
In some preparations we ocassionally observed episodes of polyVT where
the computed ECGs displayed the undulating pattern of peak-to-peak amplitude
see in Torsade de Pointes (TdP). Figure 8 and table 4 illustrates an episode
of polyVT that displays the TdP pattern which we analyzed in detail. In
this example, we also observed changes in propagation that were consistent
with discontinuous propagation.
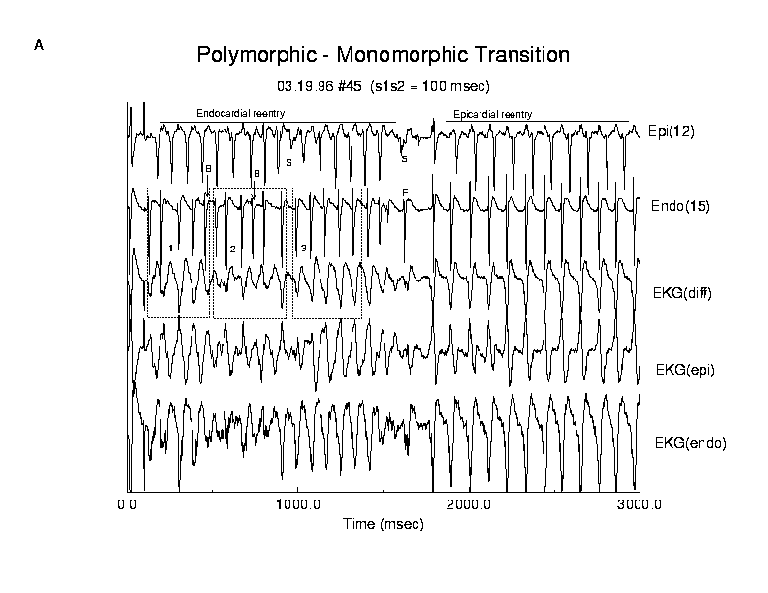
In this example, an endocardial reentry circuit formed immediately following test stimulation 100 ms after the last basic stimulus. Activation of the epicardial surface occured via transmural paths of variable delay. For the first 11 epicardial reentrant cycles, initial activation of the epicardial surface occurred with increasing delays in transmural conduction until an endocardial activation was blocked. The progression of endo-epi delays can be readily seen in figure 7. We have identified 3 regions of interest labelled 1, 2 and 3. The two blocked events are labeled "B". During TdP cycle 1, comparing the electrogram complexes in epicardial lead 12 and endocardial lead 15, we found delays of 45, 56, 46, 60, 64 ms followed by the blocked activation, B. This pattern of delays is similar to a Wenkebach progression and overlaps the undulating pattern of the peak-to-peak QRS amplitudes seen in the computed ECG immediately below the endocardial electrogram. Since overlap of individual electrogram complexes increases the QRS amplitude, it seems quite plausible that a Wenkeback-like progression could produce the undulating QRS amplitude patterns by progressively varying the phase between endocardial and epicardial activation complexes.
A similar, though shorter progression is seen in region 2. Here we observed endo-epi delays of 38, 51, 70 ms followed by a blocked beat. The coupling intervals for region 3 progressively increased (44, 51, 57, 57, 63 ms) and the computed ECG showed larger changes in morphology for the first 2 activations than for the next 3. This cycle was followed by termination of the endocardial circuit and initiation of an epicardial reentrant circuit near the lower right corner of the epicardial array. During the transition from polyVT to quasi-monoVT, the core drifted diagonally across the preparation and finally rotated stably near the upper left corner. In addition we observed changes in propagation consistent with discontinuous propagation. The two activation complexes labeled "S" were associated with a shift in the direction of propagation. There was also a paradoxical change in epicardial conduction before and after the switch from endocardial reentry to epicardial reentry. The epicardial reexcitation interval increased from 83 to 107.8 ms (p < .01) while the epicardial propagation increased from 88.7 to 141.6 ms (p < .01). On the other hand, endocardial propagation became more rapid, changing from 51.9 ms during polyVT to 29.7 ms after the reexcitation rate slowed (p < .01).
Discussion
Since the early reports of Dessertenne (16) there has been considerable interest in the origins and mechanisms of polymorphic ventricular tachycardias. At least in some cases, polyVT appears to be a two step process: an initiating process, usually thought to be an early afterdepolarization and a maintenance process: either 1) reentry or 2) either sustained activity from one or more "mobile" EAD sites (7) or competition between 2 EAD sites that activate at different rates (17). In favor of the reentry process are early studies by Horowitz et. al. (4) where programmed stimulation was used to initiate POLYVT in patients. More recently El-Sherif et. al. (7) have shown in a canine preparation that reentry was often initiated by focal activity secondary to altered repolarization currents in the presence of the neurotoxin anthopleurin A (which prolongs the action potential duration by delaying Na channel inactivation). For purposes of this discussion, we will focus our attention to mechanisms for altering QRS morphology during reentrant arrhythmias.
Our observations suggest the following mechanism for altering QRS morphology during reentry. Cycle to cycle alterations in QRS complexes arise from cycle to cycle variations in the sequence of ventricular activation. These variations in propagation produce variations in electrogram activation complexes which comprise the QRS. Large variations in the overlap of electrogram activation complexes, whether from different locations associated with the same wave or from complexes associated with two successive waves produce readily observable changes in QRS complexes. The central question is what are the mechanisms underlying variations in myocardial activation?
Recently, Kukushkin et. al. obtained nearly complete spatial-temporal potential distributions during reentry by using simultaneous epicardial and endocardial mapping (18). They identified many parameters characterizing propagation that were associated with alterations in ECG morphology. Highlighting the importance of mapping the complete surface of the preparation (in this case, the endocardial and epicardial surface) in determining the QRS morphology, they showed that slow, unstable activation on one surface (resulting in a polymorphic electrogram derived from that surface) of the preparation had only a small influence on QRS morphology if it occurred in the presence stable activation on the opposite surface (resulting in a monomorphic electrogram derived from that surface). The resulting computed ECG, computed from both endocardial and epicardial electrograms was quasimonomorphic in nature, and did not reveal the complex patterns of propagation that existed in some regions of the preparation. In such situations, the apparent reentry rate, derived from the ECG was often different from the actual reentry rate derived from the maps.
Evidence of Discontinuous Propagation
Our previous numerical experiments (5) revealed that complex polymorphic QRS complexes could be derived from spiral wave reentry in a homogeneous medium. Although our numerical studies revealed wave overlap (see figure 9 in ref 5) we did not appreciate its significance at that time. These studies were performed in a continuous medium and consequently, none of the alterations in conduction due to discontinuous propagation were observed, i.e. the inverse relationship between recovery interval and peak-to-peak electrogram amplitude and slowing of conduction with increased recovery time.
In order to more clearly understand the limitations of a continuous
medium, we performed several numerical experiments on a two dimensional
array of ventricular cells using the Beeler-Reuter model (19). Specifically,
we wanted to explore the role of overlap of electrogram complexes during
stable (monomorphic) and unstable (polymorphic) reentry (figure 9).
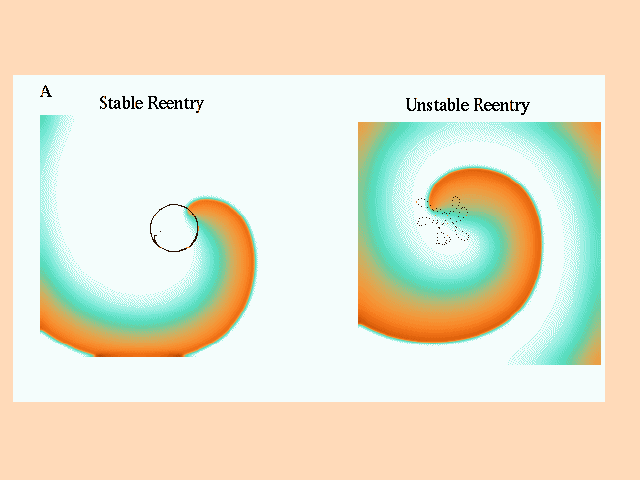
In these studies, the value of the maximum sodium conductance, gNa, was used to alter the stability of the location of the functionally unexcited core. On the left of panel A is shown an episode of stable (gNa = 2.2 mS/cm**2) reentry (the tip trajectory of successive reentrant cycles coincides) and on the right is shown an episode of unstable reentry (gNa = 3.0 mS/cm**2). The associated electrograms, measured at the center of the preparation, and at the midpoints between the center and each edge as well as the computed-ECG were computed.
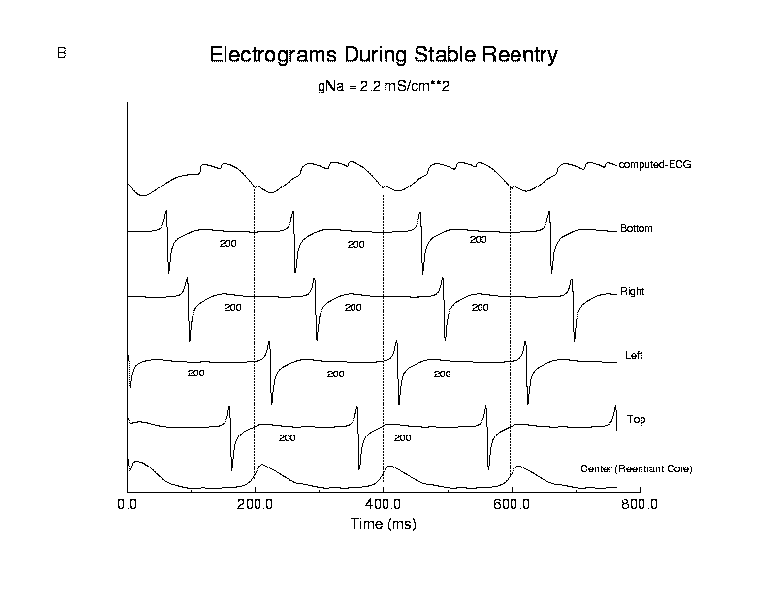
During stable reentry (panel B), the reexcitation interval was constant
(200 ms) in all computed electrograms and the peak to peak amplitude did
not vary from one cycle to the next. The relative location of each activation
complex remained fixed from cycle to cycle with the result that the computed-ECG
displayed monomorphic activation complexes. The reentrant wave rotated
about a circular core region that contained the "Center" electrode which
measured only slow changes in potential, consistent with small, subthreshold
electrotonic currents flowing from the front.
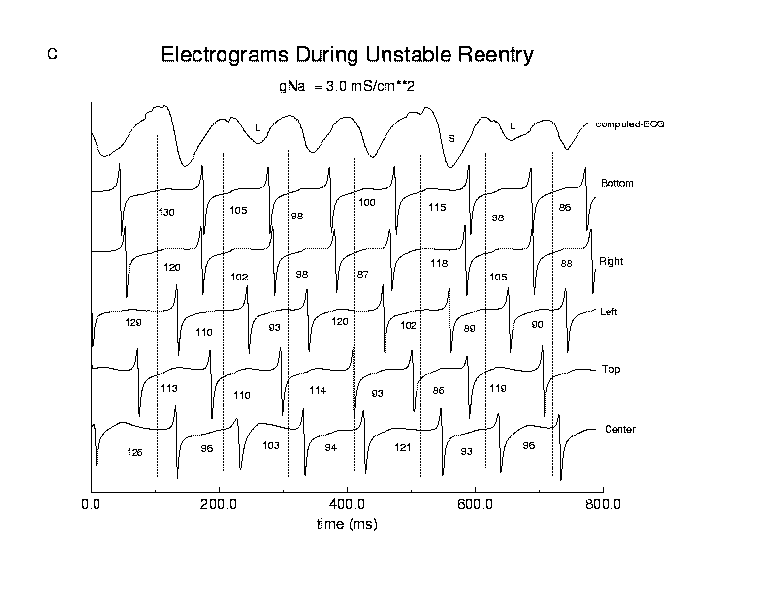
In contrast, during unstable reentry (panel C), the mean reexcitation interval was quite variable with a mean interval of 102 ms and a standard deviation of 19.5 ms. The variability in reexcitation interval produced variations in the degree of overlap of electrogram complexes, readily seen in the complexes from the Top, Bottom and Right electrodes. Note that the computed-ECG shows a maximum when there is a high degree of overlap and much smaller peak-to-peak amplitude when the activation complexes during a reentrant cycle are temporally remote, one from another. In addition, there was a positive correlation between reexcitation interval and the peak to peak amplitude of the next complex, e.g. in the Top electrogram, compare the amplitudes following 86 and 199 ms reexcitation intervals and in the Center electrode, compare the amplitudes following 96 and 103 ms reexcitation intervals. Short reexcitation intervals produced smaller amplitude complexes than longer reexcitation intervals, as predicted from cable theory and the recovery kinetics of the sodium channel.
Discontinuous propagation was evident from detailed analysis of individual electrogram activation complexes. Consider the endocardial electrograms shown in figure 5, sequence A-E of the endo:2 electrogram). It is well known that the peak-to-peak amplitude of electrogram complexes is proportional to the conduction velocity, i.e. increases in conduction velocity result in an increase in the peak-to-peak amplitude of an activation complex (12,13,20). In this example, there was a concomitant increase in the interval between successive activations (approximating the recovery interval) and the peak-to-peak amplitude of each successive complex except for complex E, where the prior interactivation interval increased from 85 ms to 93 ms but the amplitude decreased by approximately 50%.
Wenkebach Conduction and Torsade de Pointes
The mechanism of the undulating pattern of QRS amplitude (torsade de pointes) has been a source of great curiosity. Two primary candidates have been 2 competing automatic foci with slightly different firing rates (3) and unstable reentry (meandering) (5,21). In our studies, we have observed a third mechanism, that of transmural Wenkebach conduction (figure 9). The first cycle of undulation in QRS amplitude (seen in the computed ECGdiff) was associated with 5:4 block where each endo-epi coupling interval increased until block occurred. This sequence was followed by an episode of 4:3 block. On the basis of this observation, we propose a third possible mechanism for Torsade de Pointes: during endocardial or epicardial reentry, there is transmural Wenkebach conduction from either an epicardial or endocardial reentrant circuit to the contralateral surface. The progressive prolongation of endo-epi (or the reverse) conduction produces a progressive increase in overlap between endocardial and epicardial activation patterns, producing a repetitive sequence of altered QRS morphologies that is determined by the Wenkebach conduction pattern.
Conclusions
In conclusion, we have observed many episodes of reentrant arrhythmias where variations in QRS morphology were linked to variations in patterns of reentrant wave propagation. When wave propagation was stable, i.e. the variation in cycle to cycle propagation was < 10%, there was no overlap in successive activation waves and only monoVT was observed. When wave propagation was unstable, polymorphic QRS complexes were observed in the computedECG. Spontaneous conversion of polyVT to monoVT was always accompanied by stabilization of wave propagation (a reduction in the degree of variability of parameters describing myocardial activation) and a prolongation of the reexcitation interval. We found evidence of discontinuous propagation and hypothesize that this was a major determinant of the variability in observed activation patterns. Of the parameters used to characterize macroscopic wave propagation, we identified a new important predictor of QRS morphology, wave overlap, which reflects the contribution of overlapping waves to the QRS complex. These studies provide a new framework for probing the complex nature of polymorphic reentrant arrhythmias.
Acknowledgements:
We want to thank Dr. E.V. Melnikova and Professor A. Yu. Budantsev for helping with the histologic experiments and interpreting the results. We especially thank Dr. M. S. Spach for pointing out the evidence of discontinuous propagation and assisting one of us (CFS) in interpreting the individual electrograms.
- Nguyen P.T., Scheinman M.M. and Seger J. Polymorphic ventricular tachycardia: clinical characterization, therapy and QT interval. Circulation 1986; 74:340-349.
- Jackman WM, Friday KJ, Anderson JL, Aliot EM, Clark J, Lazzara R. The long QT syndromes: a critical review, new clinical obersations and a unifying hypothesis. Prog Cardiovasc Dis. 1988; 31:115-172.
- D?Alnoncourt CN, Zierhut W, Luderitz B. Torsade de pointes tachycardia: re-entry or focal activity? Br Heart J. 1982; 213-216.
- Horowitz LN, Greenspan AM, Spielman SR, Josephson ME. Torsade de pointes: electrophysiologic studies in patients without transient pharmacologic or metabolic abnormalities. Circulation, 1981; 63: 1120-1127.
- Starmer CF, Romashko DN, Reddy RS, Zilberter YI, Starobin J, Grant AO, Krinsky VI. Proarrhythmic response to potassium channel blockade: Numerical studies of polymorphic tachyarrhythmias. Circulation, 1995; 92:595-605.
- El-Sherif N, Zeiler RH, Craelius W. Gough WB, Henkin R. QTU prolongation and polymorphic ventricular tachyarrhythmias due to bradycardia-dependent early afterdepolarization. Circ Res 1988; 63:286-305.
- El-Sherif N, Caref EB, Yin H, Restivo M. The electrophysiological mechanism of ventricular arrhythmias in the Long QT Syndrome: Tridimensional mapping of activation and recovery patterns. Circ Res 1996; 79:474-492.
- Pertsov AM, Davidenko JM, Salmonsz R. Baxter WT, Jalife J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ Res 1993; 72:631-650.
- Gray RA, Jalife J, Panfilov A, Baxter WT, Cabo C, Davidenko JM, Pertsov AM. Nonstationary vortexlike reentrant activity as a mechanism of polymorphic ventricular tachycardia in the isolated rabbit heart. Circulation 1995; 91:2454-2469.
- Carlsson L, Almgren O Duker G. QTU-prolongation and torsades de pointes induced by putative class III antiarrhythmic agents in the rabbit: etiology and interventions. J Cardiovasc Pharmacol 1990; 16:276-285.
- Fast VG and Pertsov AM. Shift and termination of functional reentry in isolated ventricular preparations with quinidine-induced inhomogeneity in refractory period. J. Cardiac Electrophysiology. 1992; 3:255-265.
- Spach MS, Miller III WT, Dolber PC, Kootsey J M, Sommer JR and Mosher CE. The functional role of structural complexities in the propagation of depolarization in the atrium of the dog-cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ Res 1982; 50:175-191.
- Spach, MS, Dolber, PC and Heidlage, JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle: A model of reentry based on anisotropic discontinuous propagation. Circ. Res. 1988; 62:811-832.
- Titomir LI. Electrical Generators of the Heart. Hauka Press, Moscow, 1980.
- Sarancha, DYu, Medvinsky AB, Kukushkin NI, Sidorov VV, Romashko DN, Burashnikov AYu, Moskalenko AV, Starmer CF. A system for computer-aided visualization of propagation of excitation waves in myocardium. Biofizika 1997; 42: 502-507.
- Dessertenne, F. La tachycardie ventriculaire a deux foyers opposes variable. Arch Mal Coeur. 1966; 59:263-272.
- Bardy, GH, Ungerleider, RM, Smith, WM, Ideker, RE. A mechanism of torsade de pointes in a canine model. Circulation 1983; 67:52-59.
- Kukushkin NI, Sidorov, VYu., Medvinsky AB, Romashko DN, Burashnikov AYu, Starmer C, Sarancha D. Slow excitation waves and mechanisms of polymorphic ventricular tachycardia in an experimental model of isolated wall of the right ventricle from rabbit and suslik. Biofizika 1997; (in press).
- Beeler, GW, Reuter, H. Reconstruction of the action potential of ventricular myocardial vibers. J. Physiol. (London) 1977; 268:177-218.
- Spach, MS, Miller, WT, Geselowitz, DB, Barr, RC, Kootsey, JM and Johnson, EA. The discontinuous nature of propagation in normal canine cardiac muscle: Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res. 1981; 48:39-54.
- Winfree, AT. Electrical instability in cardiac muscle: phase singularities and rotors. J. Theor. Biol. 1989; 138:353-405.
- Starmer, CF, Biktashev, VN, Romashko, DN, Stepanov, MR, Makarova, ON and Krinsky, VI. Vulnerability in an excitable medium: Analytical and numerical studies of initiating unidirectional propagation. Biophysical J 1993; 65:1775-1787.
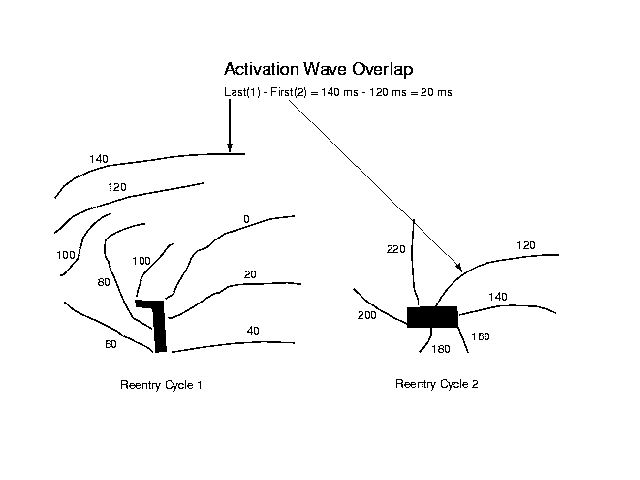
Figure 1. Wave overlap occurs when two waves of actiavtion coexist during part of a reentrant cycle and is caused by the relative relationship between the propagation time of the activation wave and the reentry cycle length. Variations in the overlap of two successive activation waves produces changes in the computed ECG that produce polymorphic configurations. Here two maps illustrate overlap of successive activation waves. During each reentrant cycle, the activation wave propagates across the preparation. If the propagation is incomplete before initiation of the next cycle, then there is overlap as shown here. For this example the last activation isochrone occurs at 140 ms while the next reentrant cycle starts at 120 ms. The large currents that flow during the initial moments of activation cause variations in wave overlap which alter the QRS morphology.
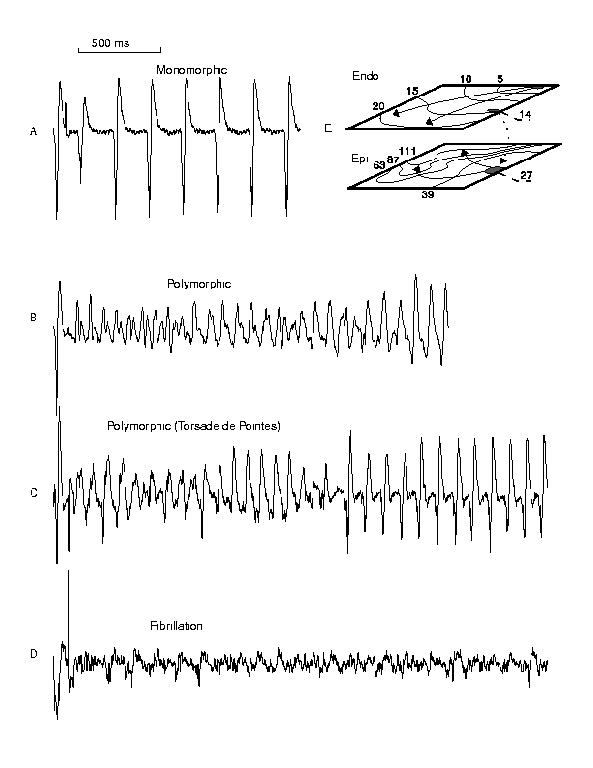
Figure 2. Typical responses to premature stimulation. Shown are the computed ECGs for examples of monomorphic reentry (A), polymorphic reentry (B), Torsade de Pointes (C) and something similar to fibrillation (D). Panel E illustrates a pair of endo- and epicardial activation maps. Each arrhythmia was initiated by a single premature stimulus and its reentrant nature was confirmed by activation maps.
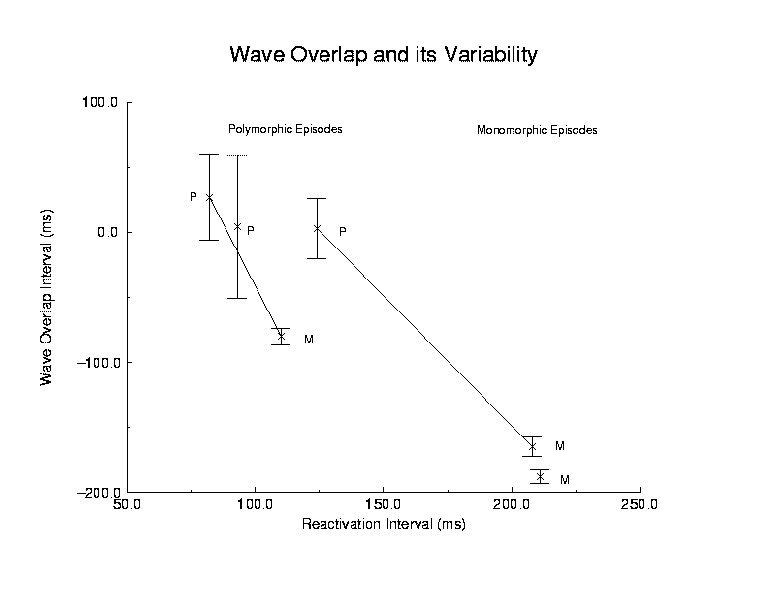
Figure 3. Polymorphic reentry was characterized by large variations in all measured parameters while monomorphic reentry was characterized by small variations in all measured parameters. Shown here is the relationship between reentry cycle length and wave overlap for polymorphic (P) and monomorphic (M) episodes described later. The lines represent a spontaneous transition from polymorphic to monomorphic reentry. The other two episodes represent continuous polymorphic or continuous monomorphic reentry.
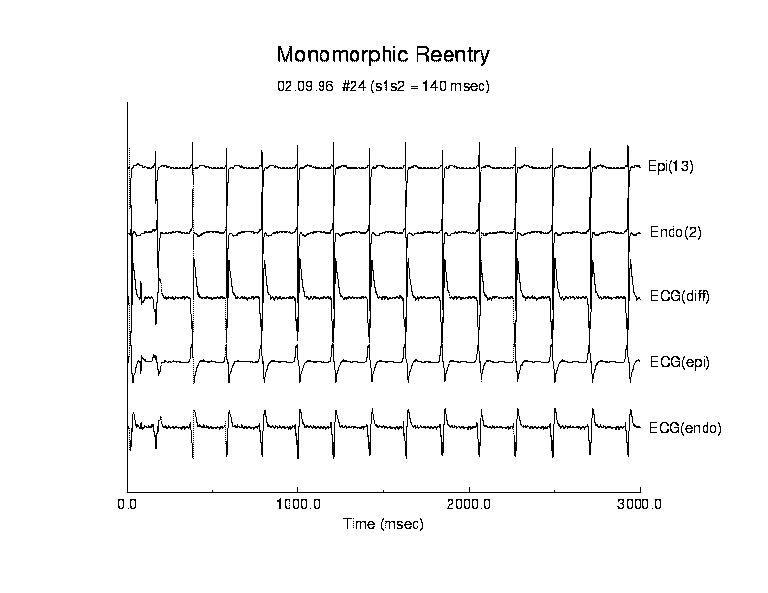
Figure 4. Activation patterns for a typical example of monomorphic reentry. Following premature stimulation, a stable transmural reentrant circuit was established. The reentrant circuit remained stationary throughout this episode. The stability of cycle-to-cycle propagation was reflected by the small variance of all measured parameters (table 1).
Figure 5. Activation patterns for a typical example of polymorphic reentry. Following premature stimulation, an endocardial reentrant circuit was established that supported rapid propagation. Evidence of discontinuous propagation was observed in the endocardial electrogram (electrode 2, sequence labeled A?B? and ABCDE. In the first sequence, the interval prior to activation of A? was 79 ms while the interval prior to activation of B? was 100 ms. Note that the peak to peak amplitude of B? is less than that of A? even though there was a longer recovery interval. Similar effects are seen in the sequence ABCDE. The intervals preceding E are: AB = 81ms, BC = 82 ms, CD = 85 ms and DE = 93 ms. Even though the DE interval is longer than the prior intervals, the amplitude of E is much less indicating a change in propagation direction and perhaps velocity. Such alterations produced large variations in all measured parameters consistent with the observed variations in wave overlap.
Figure 6. Electrograms for an episode of polyVT followed by spontaneous transition to monoVT. Following premature stimulation, an unstable endocardial reentry circuit was formed that produced a polymorphic computed ECG. After approximately 2 seconds, the reentry circuit shifted to the epicardial surface and the reentry interval slowed from 124 ms to 207 ms with a concommitant reduction in variability.
Figure 7. Maps illustrating the small degree of variable wave overlap during polyVT and negative overlap (no overlap) with minimal variability during monoVT. In this example, during 2 successive polymorphic cycles there was 31 and 27 ms of overlap in endocardial activation and 13 and ?19 ms of overlap in epicardial activation. In addition, there was drift in the unexcited core as shown in the endocardial maps. During monoVT, though, the overlap was ?168 and ?169 ms in endocardial activation and ?45 and ?48 ms in epicardial activation. Moreover, the unexcited core was relatively stable as seen in the epicardial maps.
Figure 8. Activation patterns and ECGs before and after spontaneous conversion from polyVT to qmonoVT. Immediately after test stimulation, an endocardial reentrant circuit formed and there was a progressive delay between the endocardial and epicardial excitation which resulted in blocked endocardial activation (labeled B in endo electrode 15 electrogram). The Wenkebach-like transmural conduction paralleled the undulating pattern of torsade de points in the computed ECG (see regions 1, 2 and 3). There was also evidence of transient changes in epicardial propagation (labeled S in epi electrode 12) consistent with a shift from longitudinal to transverse conduction. After approximately 1.5 seconds of polyVT propagation was altered in a manner that lead to the formation of an epicardial reentrant circuit and a more monomorphic pattern of electrogram and ECG complexes. During the transition, the endocardial and epicardial electrograms show opposing effects of extended recovery. The epicardial complex (labeled S) is of reduced amplitude even though the prior recovery time is longer (124 ms) than that of the previous excitation (91 ms) while the endocardial complex (labeled F) has a larger amplitude than that of previous complexes. Note that the torsade de pointes pattern observed during the first 0.5 seconds was also associated with changing patterns of wave overlap, predominantly displayed in the ECGdiff. During the TdP cycle, complexes in the ECGendo or ECGepi showed less dramatic changes in morphology. The ECGdiff illustrates summation of the negative peaks in the endocardial complexes which were temporally aligned with the positive peaks of the epicardial complexes which resulted in the large peak to peak complexes seen in the ECGdiff. In this episode, the variations in wave overlap were associated with Wenkebach conduction between endocardial and epicardial activation. This example also illustrates the importance of including both endocardial and epicardial activity in computing the ECG since neither epicardial and endocardial summations of electrograms adequately represent the complexity of propagation on both surfaces.
Figure 9. Stable and unstable reentry in a continuous medium. Panel A displays a reentrant (spiral) wave and the trajectory followed by the tip of the wave during stable (left) and unstable (right) reentry. A 7.5 cm x 7.5 cm two dimensional array of ventricular cells (using the Beeler-Reuter (19,22) model) was used for numerical experiments. Electrograms were computed at sites at the center of the preparation (x = L/2, y = L/2) and between the center and each edge (Top = (L/2,L/4); Left = (L/4,L/2); Right = (3L/4, L/2); Bottom = (L/2, 3L/4)). Panels B and C show computed electrograms and the computed-ECG at these 5 locations. During stable reentry (Panel B, reexcitation interval = 200 ms), there was no variation in reexcitation interval (note the fixed position of electrogram activation complexes relative to 200 msec dashed lines) and peak to peak electrogram amplitude. The functionally unexcited core contained the center electrode, which only showed signs of slow changes in membrane potential, consistent with subthreshold electrotonic currents flowing from nearby excited cells. During unstable reentry (Panel C, reexcitation interval = 102 +/- 19.5 ms) there is considerable variability in the overlap of activation complexes. During the reentrant cycle starting at approximately 400 ms, the electrogram from the top electrode shows two activations within the same cycle, i.e. overlap of two activation waves. Note the variations in location of activation complexes across different 102 ms intervals. The computed-ECG shows maximum amplitude when the time between electrogram complexes (overlap) different leads is small (labeled S) and shows smaller amplitude complexes when there are larger intervals (labeled L) between activation complexes in different leads, similar to that seen in our experimental preparations. In addition, there was a direct correlation between peak to peak electrogram amplitude and the preceding reexcitation interval, e.g. long intervals were associated with high amplitude complexes whereas shorter intervals were associated with lower amplitude complexes. This is a result of variations in sodium channel recovery in a continuous medium. This numerical result conflicts with our experimental observations and argues against a virtual syncytium and in favor of discontinuous propagation.
Table 1: Propagation Parameters During Monomorphic Reentry:
Reexcitation Interval 211.
5.0
mono endo
212.
4.7
mono epi
Activation Envelope 22.8
0.9
mono endo
96.6
3.7
mono epi*
Wave Overlap
-188.
5.3
mono endo
-115.
8.1
mono epi*
Transmural Delay 27.8 0.6 mono
*endo significantly different from epi (p < .01)
Table 2: Propagation Parameters During Polymorphic Reentry:
Parameter Mean(ms) Std. Dev.(ms) Type Surface
98.5 36.7 poly epi
Activation Envelope 83.6
24.9
poly endo
41.3
poly epi
Wave Overlap
4.46
55.1
poly endo
2.85
50.8
poly epi
Transmural Delay 45.0 42.5 poly
Table 3: Propagation Parameters During Poly- and Monomorphic Reentry:
Study 02.08.96 #43
Parameter Mean(ms) Std. Dev.(ms) Type Surface
Reexcitation Interval 124.
31.7
poly endo
207.
1.9
mono* endo
122.
25.0
poly epi
208.
2.5
mono* epi
Activation Envelope 122.
39.5
poly endo
39.1
0.4
mono* endo
111.
30.7
poly epi
161.
3.0
mono* epi +
Wave Overlap
3.0
58.0
poly endo
-165
7.6
mono* endo
-10.6
29.6
poly epi
-161
9.2
mono* epi
Transmural Delay
-6.4
16.7
poly
21.0
9.7
mono
* poly significantly different from mono (p < .01)
+ endo significantly less than epi (p < .01)
Table 4: Propagation Parameters during Poly- (TdP) and Quasimonomorphic Reentry:
Study 03.19.96 #45
Parameter
Mean(ms)
Std. Dev.(ms) Type
Surface
Reexcitation Interval
82.5
17.9
poly
endo
109.5
9.5
mono* endo
83.0
26.9
poly
epi
107.8
7.3
mono* epi
Activation Envelope 51.9
18.4
poly
endo
29.7
5.6
mono* endo
88.7
25.5
poly
epi
141.6
4.4
mono* epi +
Wave Overlap
27.5
23.6
poly
endo
-79.2
6.2
mono* endo
4.1
33.5
poly
epi
32.6
9.7
mono* epi +
Transmural Delay
-26.5
24.3
poly
-29.6
4.4
mono*
* poly significantly different from qmono (p < .01)
+ endo significantly less than epi (p < .01)