Articles |
From the Departments of Medicine (Cardiology) and Computer Science, Duke University Medical Center, Durham, NC (C.F.S., Y.I.Z., J.S., A.O.G.); Department of Biomedical Engineering, Indian Institute of Technology–Madras, India (C.F.S., R.S.R.); Institute of Theoretical and Experimental Biophysics, Pushchino, Moscow Region, Russia (D.N.R., V.I.K.); and Institut Non-Lineaire de Nice, Valbonne, France (V.I.K.).
Correspondence to C. Frank Starmer, Box 3181, Duke University Medical Center, Durham, NC 27710. E-mail cfs@rodney.mc.duke.edu.
| Abstract |
|---|
|
|
|---|
Methods and Results To assess the generic role of repolarizing currents during reentry, we studied the responses of a two-dimensional array of identical excitable cells based on the FitzHugh-Nagumo model, consisting of a single excitation (sodium-like) current and a single recovery (potassium-like) current. Spiral wave reentry was initiated by use of S1S2 stimulation, with the delay timed to occur within the vulnerable period (VP). While holding the sodium conductance constant, the potassium conductance (gK) was reduced from 1.13 to 0.70 (arbitrary units), producing a prolongation of the action potential duration (APD). When gK was 1.13, the tip of the spiral wave rotated around a small, stationary, unexcited region and the computed ECG was monomorphic. As gK was reduced, the APD was prolonged and the unexcited region became mobile (nonstationary), such that the tip of the spiral wave inscribed an outline similar to a multipetaled flower; concomitantly, the computed ECG became progressively more polymorphic. The degree of polymorphism was related to the APD and the configuration of the nonstationary spiral core.
Conclusions Torsadelike (polymorphic) ECGs can be derived from spiral wave reentry in a medium of identical cells. Under normal conditions, the spiral core around which a reentrant wave front rotates is stationary. As the balance of repolarizing currents becomes less outward (eg, secondary to potassium channel blockade), the APD is prolonged. When the wavelength (APD · velocity) exceeds the perimeter of the stationary unexcited core, the core will become unstable, causing spiral core drift. Large repolarizing currents shorten the APD and result in a monomorphic reentrant process (stationary core), whereas smaller currents prolong the APD and amplify spiral core instability, resulting in a polymorphic process. We conclude that, similar to sodium channel blockade, the proarrhythmic potential of potassium channel blockade in the setting of propagation may be directly linked to its cellular antiarrhythmic potential, ie, arrhythmia suppression resulting from a prolonged APD may, on initiation of a reentrant wave front, destabilize the core of a rotating spiral, resulting in complex motion (precession) of the spiral tip around a nonstationary region of unexcited cells. In tissue with inhomogeneities, core instability alters the activation sequence from one reentry cycle to the next and can lead to spiral wave fractionation as the wave front collides with inhomogeneous regions. Depending on the nature of the inhomogeneities, wave front fragments may annihilate one another, producing a nonsustained arrhythmia, or may spawn new spirals (multiple wavelets), producing fibrillation and sudden cardiac death.
Key Words: reentry • arrhythmia • potassium • sodium • tachycardia
| Introduction |
|---|
|
|
|---|
As consideration of potassium channel blockade by class III agents increased, we began to use similar numerical and in vitro studies to explore the antiarrhythmic and potentially proarrhythmic potential of potassium channel blockade. In a general sense, sodium and potassium currents are complementary: one depolarizes, the other repolarizes. With sodium channel blockade, the cellular antiarrhythmic property (prolongation of recovery of excitability) leads to a multicellular proarrhythmic property (prolongation of the VP) that destabilizes bidirectional wave front formation. Similarly, with potassium channel blockade, the cellular antiarrhythmic property derived from prolongation of the action potential duration (APD) (prolonged refractoriness) might similarly translate to a multicellular proarrhythmic property by prolonging the wavelength (APD · velocity) and destabilizing some other aspect of propagation.
Studies of the antiarrhythmic and proarrhythmic responses to sodium channel blockade were facilitated by a well-accepted model of reentrant arrhythmias.6 Unidirectional block of the wave front secondary to premature stimulation was accepted as the necessary prerequisite for initiating a reentrant arrhythmia. Although there is an association of torsade de pointes with actions that alter the balance of repolarizing currents (such as potassium channel blockade or hypokalemia) and more generally with polymorphic processes,7 there is no widely accepted model of initiation or of maintenance of torsadelike arrhythmias from which one may develop a measure of the proarrhythmic potential of a drug.
For exploring reentrant arrhythmias in the setting of reduced potassium currents, we have temporarily delayed considering the mechanistic basis of the event that initiates reentrant activity, hypothesizing that for some situations, the "initiating" and "maintenance" processes are distinct and can be explored separately. For torsadelike arrhythmias (polymorphic tachyarrhythmias), for instance, there appear to be several different modes of initiation. Some torsadelike arrhythmias are initiated by early afterdepolarizations (EADs) during bradycardiac conditions: either a regular bradycardia or a transient pause before the initiating event.7 However, recent studies also indicate that torsadelike arrhythmias can be associated with a short coupling interval between the preceding beat and the first element of the arrhythmia.8 Once initiated, if conditions suitable for maintaining tachycardia are encountered, then one or more cycles of polymorphic complexes might occur. The mechanistic nature of both initiation and maintenance of the tachyarrhythmia, however, is uncertain. Two competing automatic regions of slightly different rate have been proposed.9 It is also possible that the arrhythmia is self-reactivating and reentrant in nature.10 Our studies have focused on the arrhythmia-maintenance process and have assumed that at least some variants of polymorphic tachyarrhythmias are reentrant.
Polymorphic tachyarrhythmias are sometimes thought to be incompatible with a reentrant process because in the setting of quinidine and other class Ia drugs, it is difficult to induce sustained tachycardia with the use of programmed stimulation.11 Recently, however, we showed in both numerical4 and experimental3 studies of quinidine that potassium channel blockade reduces the VP during which reentrant rhythms can be initiated. Thus, the results of these clinical studies could reflect deficiencies of the stimulation protocol. More specifically, programmed stimulation may fail to initiate a sustained arrhythmia if the step size in S1S2 delay exceeds that of the VP. Similarly, if a reentrant process can be initiated, the point of reentry might move in a manner that limits the duration of the arrhythmia.10
The reentrant wave front recently has been directly visualized using tissue slices and shown to be that of a spiral wave.12 These spiral waves have been observed to either rotate around a small core region or to drift in response to local inhomogeneities in the medium. Theoretical studies have shown that properties of the medium can influence stability of motion of the spiral tip,13 14 and under certain conditions, tip motion will be reflected in the ECG. If the spiral tip rotates around a stationary region, the activation wave front will follow the same path from one complete reentrant cycle to the next and the associated ECG will exhibit a monomorphic pattern. When the tip rotates about a nonstationary region15 then the activation sequence from one reentrant cycle to the next will be different and the associated ECG can exhibit polymorphic patterns.
Here we report that polymorphic ECGs arise during reentry in a continuously uniform medium when repolarization (APD wavelength) is prolonged. For purposes of demonstration, prolongation of the APD was achieved by reducing the magnitude of a generalized potassium conductance, mimicking either channel blockade16 or hypokalemia,17 although similar results could be achieved by any intervention that prolonged the APD.
We used the FitzHugh-Nagumo18 cellular model, based on a single excitation (sodium) and repolarizing (potassium) current, to probe the "generic" reentrant behavior of an array of excitable cells without having to deal with the multiple inward or outward currents that are present in cardiac cells. Our goal was to identify the minimally complex membrane model that could lead to instability of spiral reentry resulting in polymorphic ECGs.
Because of the large number of potassium channels that are active in cardiac membrane, one might question the utility of studies of an admittedly simplistic membrane model. With respect to spiral wave stability, we have first focused our hypotheses not on the role of individual channels that contribute to membrane current during repolarization but rather on the more general relation between the action potential wavelength (as determined by the APD) and the perimeter of the unexcited spiral core. Our "repolarizing current" thus represents the sum of all the different inward and outward currents that are active during repolarization in real cardiac cells. Results from such an analysis can provide generic insights into seemingly complex polymorphic processes and give some direction to developing pharmaceutical agents that exhibit a combination of effects. For instance, while potassium channel blockade will prolong the APD, so will ß-stimulation (via an increased calcium current during repolarization) and use of a sodium channel opener. Thus, if one wanted to develop a compound that reduced the proarrhythmic potential associated with APD prolongation, one might design a drug that would block a major potassium repolarizing current, ß-receptors (to minimize sympathetic modulation), and calcium channels to minimize the possible inward currents necessary for EAD production. In subsequent studies, we plan to increase the complexity of the mix of transmembrane currents and evaluate whether these components of the repolarizing process amplify or attenuate the underlying proarrhythmic substrate.
Our basic finding was that as reentrant activation progresses in a homogeneous array of identical cells in which repolarizing currents are reduced, the tip of the spiral wave becomes unstable, and instead of rotating around a small stationary core, the core becomes nonstationary and the tip inscribes the outline of a multipetaled flower. The size of the flower and the number of petals depend on the balance of inward and outward currents during repolarization. Spiral core stability is reflected in the ECG by the number and amplitude of oscillating patterns in one polymorphic cycle. Rapid repolarization results in a relatively stationary core (small flower), whereas prolonged repolarization results in a nonstationary core (large flowers). The ECG computed at a site near the spiral core associated with a stationary core displays a monomorphic pattern, whereas the computed ECG associated with a large flower is polymorphic and similar to that seen in torsade de pointes syndrome.
Our results suggest that potassium channel blockade, in addition to reducing cardiac vulnerability and suppressing premature excitation by prolonging the APD, can, if spiral reentry is initiated, destabilize the core region of the spiral such that the tip tends to wander around the myocardial surface. Extrapolations from our studies to an inhomogeneous medium suggest that nonsustained tachycardias and ventricular fibrillation seen in patients with long QT syndrome or hypokalemia or in the presence of potassium channel blockade are compatible with a reentrant process in the setting of diminished repolarizing currents. Reentry may be terminated as a result of large excursions of the spiral tip, which force the wave front into an inexcitable region. An inexcitable region can also break incident wave fronts in a manner that permits development of additional spiral waves19 that collectively can lead to fibrillatory-like arrhythmias and sudden cardiac death.
| Methods |
|---|
|
|
|---|
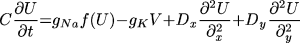 |
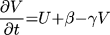 |
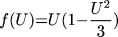 |
where t is time, x and y are spatial
coordinates, U(x,y,t) is the transmembrane potential,
V(x, y, t) is the slow repolarizing driving force, f(U) is
the nonlinear voltage-current relation of the excitation current,
gNa and gK are pseudo-sodium and -potassium
conductances, ß and  are parameters defining the
recovery kinetics, and C is the membrane capacitance.
are parameters defining the
recovery kinetics, and C is the membrane capacitance.
The equations were integrated with an explicit method by use of spatial
grids ranging in size from 30x30 to 90x90. A two-point difference
was
used to approximate the time derivative, and a three-point difference
was used to approximate spatial derivatives. In the numerical
experiments, the model parameters were ß = 0.6,  = 1,
c = 0.025, Dx = Dy
= 0.7,
the integration time step,
= 1,
c = 0.025, Dx = Dy
= 0.7,
the integration time step,  t, ranged from 1/256 to 1/1024, and
t, ranged from 1/256 to 1/1024, and
 x =
x =  y ranged from 1 to 0.3. Because we were
primarily interested in the qualitative features of spiral wave
development and evolution, we did not scale the model
parameters to any specific set of physical units. For
studies of the spiral tip trajectory, typically a 60x60 grid was used,
where
y ranged from 1 to 0.3. Because we were
primarily interested in the qualitative features of spiral wave
development and evolution, we did not scale the model
parameters to any specific set of physical units. For
studies of the spiral tip trajectory, typically a 60x60 grid was used,
where  x=
x= y=0.5 and
y=0.5 and
 t=1/1024. Results were
tested for stability by halving time and space steps and repeating a
calibration protocol in which we initiated a spiral and monitored its
evolution over a period of five full rotations.
t=1/1024. Results were
tested for stability by halving time and space steps and repeating a
calibration protocol in which we initiated a spiral and monitored its
evolution over a period of five full rotations.
The ECG was computed by integrating all the membrane currents,20 weighted by the distance from a remote observation point:
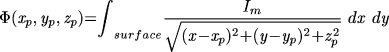 |
where
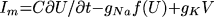 |
Two "extremity" ECGs were computed to the left and right of the sheet of cells and one "precordial" lead was computed near the center of the sheet. Computations were performed on an AST 386/SX20 and SUN SPARC 2 using programs written in C. All results are reported in nondimensional units (arbitrary time units, ATU).
To confirm results obtained from the computationally simple FitzHugh-Nagumo model, we repeated measurements of spiral tip trajectories for a range of values of the inward rectifier conductance in the Beeler-Reuter21 model of a ventricular cell. A 100x100 array of excitable cells was studied using the parameters described in Reference 1. The calcium conductance was reduced by half because of wave front instabilities that arose after approximately 15 rotations. The sodium and potassium conductances (gNa and gK, respectively) were varied, movement of the spiral tip was tracked, and an ECG was computed at a point over the midpoint of the array.
| Results |
|---|
|
|
|---|
|
|
Spiral wave fronts, propagating in a clockwise manner, were initiated
by S1S2 stimuli timed to fall within the VP.
After a spiral wave front was formed, we noted that the tip of the
spiral was not stationary but moved in a quasicircular manner around
the perimeter of a "core" region. We tracked the tip of the
spiral by following the path of the unstable point (u=0, v=0).
The
trajectory followed by the tip was sensitive to the amplitude of the
repolarizing current. Fig 3A![]() and 3B
and 3B![]() illustrate
the
rotation of the spiral wave front as time progresses and the boundary
of the inexcitable core region. When gK was 1.13 (control),
the wave front rotated around a small region (Fig 3A
illustrate
the
rotation of the spiral wave front as time progresses and the boundary
of the inexcitable core region. When gK was 1.13 (control),
the wave front rotated around a small region (Fig 3A![]() ). When
gK was reduced to 0.75, the spiral wave front rotated
around a much larger region, reflecting the prolonged refractory period
of the cells that comprised the excitable medium (Fig 3B
). When
gK was reduced to 0.75, the spiral wave front rotated
around a much larger region, reflecting the prolonged refractory period
of the cells that comprised the excitable medium (Fig 3B![]() ). Fig
4A
). Fig
4A![]() and 4B
and 4B![]() illustrate the outline of the
trajectory
followed by the spiral tip. As in Fig 4A
illustrate the outline of the
trajectory
followed by the spiral tip. As in Fig 4A![]() , with a
"control"
gK (1.13), the outline of a small, four-petaled flower was
inscribed during three rotations of the spiral. In contrast, when the
conductance was reduced to 0.75, six rotations of the spiral wave front
were required to inscribe a complete flower (Fig 4B
, with a
"control"
gK (1.13), the outline of a small, four-petaled flower was
inscribed during three rotations of the spiral. In contrast, when the
conductance was reduced to 0.75, six rotations of the spiral wave front
were required to inscribe a complete flower (Fig 4B![]() ).
).
|
|
The movement of the spiral tip was reflected in the computed ECGs (Fig
5![]() ). In Fig 5
). In Fig 5![]() , when gK was 1.13, the
computed
ECG was similar to a monomorphic tachycardia (Fig 5A
, when gK was 1.13, the
computed
ECG was similar to a monomorphic tachycardia (Fig 5A![]() , lower
tracing). However, as the gK was reduced, the morphology of
the activation complexes evolved to a polymorphic pattern and took
on the appearance of a torsadelike ECG. Each oscillation of
potential in the computed ECG was associated with one rotation of the
spiral. Fig 5B
, lower
tracing). However, as the gK was reduced, the morphology of
the activation complexes evolved to a polymorphic pattern and took
on the appearance of a torsadelike ECG. Each oscillation of
potential in the computed ECG was associated with one rotation of the
spiral. Fig 5B![]() illustrates a single flower (left) inscribed by
the
spiral tip during one torsade cycle and the pattern inscribed by the
spiral tip during 50 ATU (right).
illustrates a single flower (left) inscribed by
the
spiral tip during one torsade cycle and the pattern inscribed by the
spiral tip during 50 ATU (right).
|
The torsadelike nature of the ECG was sensitive to the size of the
excitable medium. Fig 6![]() shows ECGs computed for
gK of 0.75 ATU for a 40x40 array and a 90x90 array.
When
the size of the medium was 40x40, only a small spiral fragment formed,
so that the relative contribution of the tip region to the computed ECG
was greater than for a 90x90 array where a larger spiral was able to
form. Note that the modulation (polymorphism) of the ECG waveform
derived from the 40x40 array was much greater than that derived from
the 90x90 array.
shows ECGs computed for
gK of 0.75 ATU for a 40x40 array and a 90x90 array.
When
the size of the medium was 40x40, only a small spiral fragment formed,
so that the relative contribution of the tip region to the computed ECG
was greater than for a 90x90 array where a larger spiral was able to
form. Note that the modulation (polymorphism) of the ECG waveform
derived from the 40x40 array was much greater than that derived from
the 90x90 array.
|
During reentrant activation, the APD and time to activation at each
cell varied as a function of spiral tip movement. As shown in Fig
7![]() , the time between successive activations and the APD
oscillated in response to spiral tip movement. When the gK
was large (1.13), the amplitudes of the oscillation of the
time between successive activations and of the APD were minimal,
reflecting the fact that the spiral wave front rotated around a
relatively small region. On the other hand, as the gK was
reduced, the amplitude of the APD oscillations increased in
parallel with the oscillation of the time between
successive activations, reflecting movement of the entire spiral wave
front as the tip wandered around the medium.
, the time between successive activations and the APD
oscillated in response to spiral tip movement. When the gK
was large (1.13), the amplitudes of the oscillation of the
time between successive activations and of the APD were minimal,
reflecting the fact that the spiral wave front rotated around a
relatively small region. On the other hand, as the gK was
reduced, the amplitude of the APD oscillations increased in
parallel with the oscillation of the time between
successive activations, reflecting movement of the entire spiral wave
front as the tip wandered around the medium.
|
Fig 8![]() illustrates typical results obtained from the
Beeler-Reuter21 ventricular model. This model
incorporates channel gating and multiple ionic currents, and it is more
representative of a cardiac cell than the
FitzHugh-Nagumo model. Such models, which represent more
accurately the complexity of cardiac currents, provide a substrate for
assessing the role of various specialized currents in amplification or
attenuation of the underlying generic responses to excitation. For
instance, the Beeler-Reuter model contains both a delayed rectifier and
an inward rectifier potassium current. Our studies found that spiral
evolution was relatively insensitive to changes in the delayed
rectifier conductance but was sensitive to changes in either the
gNa or the inward rectifier conductance. Increases in
gNa produced results qualitatively similar to those
obtained by reducing gK1. Fig 8
illustrates typical results obtained from the
Beeler-Reuter21 ventricular model. This model
incorporates channel gating and multiple ionic currents, and it is more
representative of a cardiac cell than the
FitzHugh-Nagumo model. Such models, which represent more
accurately the complexity of cardiac currents, provide a substrate for
assessing the role of various specialized currents in amplification or
attenuation of the underlying generic responses to excitation. For
instance, the Beeler-Reuter model contains both a delayed rectifier and
an inward rectifier potassium current. Our studies found that spiral
evolution was relatively insensitive to changes in the delayed
rectifier conductance but was sensitive to changes in either the
gNa or the inward rectifier conductance. Increases in
gNa produced results qualitatively similar to those
obtained by reducing gK1. Fig 8![]() shows the tip
trajectories
and associated ECGs for gK1 of 0.35 and 0.31 and
gNa of 2.2. The results are similar to those obtained from
the FitzHugh-Nagumo model, illustrating the generic nature of spiral
tip sensitivity to medium size and repolarizing currents.
shows the tip
trajectories
and associated ECGs for gK1 of 0.35 and 0.31 and
gNa of 2.2. The results are similar to those obtained from
the FitzHugh-Nagumo model, illustrating the generic nature of spiral
tip sensitivity to medium size and repolarizing currents.
|
| Discussion |
|---|
|
|
|---|
As we shifted our attention to antiarrhythmic and possibly proarrhythmic mechanisms associated with potassium channel blockade, we found our progress slowed by the absence of an accepted model of arrhythmic events derived from variations in membrane repolarization. Polymorphic tachyarrhythmias in general and, more specifically, torsade de pointes, have been associated with repolarization anomalies, yet there is no general consensus as to the underlying mechanism. Studies by Abildskov and Lux22 and Pertsov et al12 demonstrated that torsadelike polymorphic ECGs can be observed in arrays of excitable cells in which either the refractory properties were modified or gradients of excitability or conduction velocity were superimposed. Whether gradients of excitability or refractoriness were essential for polymorphic ECGs, however, was uncertain.
Krinsky and Efimov14 suggested a mechanism of a polymorphic process that linked prolongation of the cellular APD with a multicellular proarrhythmic response. Specifically, during reentry, the spiral tip migrates around a region of unexcited cells. If the wavelength of the wave front (APD · velocity) is greater than the perimeter of the unexcited region, then the spiral core (unexcited region) will become nonstationary and migrate. Such migration alters the pattern of activation from one reentry cycle to the next, thus producing a polymorphic ECG, and should be observable in a homogeneous medium devoid of spatially dispersed refractory properties.
Although the origin of the initiating events in polymorphic processes is uncertain, EADs after a pause are often associated with torsade de pointes. Under conditions of reduced currents during repolarization, the plateau potential can become unstable and oscillate, leading to EADs.28 29 EADs can occur during the VP, as defined by our earlier studies.4 5 Consequently, we hypothesize that the following sequence of events might reflect one mechanism for initiation and maintenance of a reentrant arrhythmia displaying a polymorphic ECG.
During normal sinus rhythm, the delayed rectifier potassium channels provide current for cellular repolarization. These channels have a long recovery time constant (>400 milliseconds, so that at a heart rate of 70 beats per minute, not all open channels will close during the diastolic interval. At a constant stimulus rate, the APD will achieve a steady-state value, depending on the degree of closure of these channels. If there is a long pause, then more channels will close, thus diminishing the repolarizing current available for the next action potential and prolonging the APD, promoting electrical instability and EADs, particularly in Purkinje cells. Because EADs often fall late during the action potential plateau, they may coincide with the VP.4 5
These events are displayed in
Fig 9![]() . The panels (left to
right, top to bottom) reflect a temporal sequence of views of
activation wave fronts after excitation by an EAD. We first initiated a
planar wave front propagating from left to right, shown as the vertical
color bands, with a blue leading edge. We then simulated an EAD near
the end of the action potential plateau in a small region behind the
initial wave front (blue rectangle). Because the EAD occurs during the
VP, antegrade wave front formation is inhibited and retrograde wave
front formation is permitted. This newly formed wave front fragment
propagates in the retrograde direction. The increased load "seen"
by cells at the end of the lower tip of the wave front retards
propagation of the tip, so the wave front curls and begins to form a
spiral (top row). As this fragment rotates around a core region of
unexcited cells, the spiral lengthens. Movement of the tip of the wave
front, at which the leading edge almost touches the trailing edge, is
indicated by the black lines and shows the outline of the nonstationary
core around which the spiral wavefront rotates as a result of the
prolonged APD. This sequence demonstrates how potassium channel block
can both promote an event initiating reentry (electrical instability
during the action potential plateau, leading to an EAD) and destabilize
the spiral core, leading to a polymorphic ECG.
. The panels (left to
right, top to bottom) reflect a temporal sequence of views of
activation wave fronts after excitation by an EAD. We first initiated a
planar wave front propagating from left to right, shown as the vertical
color bands, with a blue leading edge. We then simulated an EAD near
the end of the action potential plateau in a small region behind the
initial wave front (blue rectangle). Because the EAD occurs during the
VP, antegrade wave front formation is inhibited and retrograde wave
front formation is permitted. This newly formed wave front fragment
propagates in the retrograde direction. The increased load "seen"
by cells at the end of the lower tip of the wave front retards
propagation of the tip, so the wave front curls and begins to form a
spiral (top row). As this fragment rotates around a core region of
unexcited cells, the spiral lengthens. Movement of the tip of the wave
front, at which the leading edge almost touches the trailing edge, is
indicated by the black lines and shows the outline of the nonstationary
core around which the spiral wavefront rotates as a result of the
prolonged APD. This sequence demonstrates how potassium channel block
can both promote an event initiating reentry (electrical instability
during the action potential plateau, leading to an EAD) and destabilize
the spiral core, leading to a polymorphic ECG.
|
As shown, we were able to produce polymorphic electrograms from reentrant, spiral activation in a homogeneous two-dimensional array of identical cells. To our surprise, the polymorphic ECG was derived from movement of the spiral tip around a nonstationary core of unexcited cells.14 Because the spiral core was unstationary, the activation sequence varied from one reentry cycle to the next, leading to oscillation of both the time between successive cellular activations and the APD, similar to experimental results reported in other preparations.2 23
The polymorphic nature of the ECG is readily explained. The ECG represents a weighted average of the cellular membrane currents. Currents close to the monitoring electrode make a large contribution, and currents distant to the monitoring electrode make only a small contribution.20 For a spiral wave front, the cells composing the tip will have a significant effect on the ECG only when they represent a significant fraction of the cells defining the total spiral wave. In other words, if the spiral wave front is several revolutions long, then the contribution to the ECG of the cells in the tip will be negligible compared with cells in the remainder of the wave front, resulting in a monomorphic ECG. On the other hand, if only a small spiral fragment is able to develop, then the contribution of the cells in the spiral tip will be amplified, and a polymorphic ECG may result if the core region is unstable.
Spiral Wave Properties
The nature of spiral rotation is
complex, but two major factors
influence the movement of the spiral wave front: excitability and
wavelength.30 Excitability is a major determinant of the
size of the unexcited region around which the reentrant wave front
rotates, and wavelength is a major determinant of the stability of the
unexcited core. From a physiological viewpoint,
excitability is determined by the interaction between the source of
depolarizing current (sodium channel availability) and the resistance
between coupled cells (load). The greater the excitability, the easier
it is for a cell to excite neighboring cells. Consequently, in a
homogeneous medium, the radius of the unexcited core may be
small, and if the wavelength is smaller than the perimeter of the core,
then the core will be stationary and there will be an excitable gap. If
the core is stationary, then the resulting ECG will be monomorphic.
With sodium channel blockade, excitability is reduced, propagation velocity is reduced, and the radius of curvature of the core is increased (because an individual cell at the spiral tip can no longer supply the current demanded by the load presented by adjoining cells). At the cellular level, reduced excitability is antiarrhythmic. However, as discussed above, slowed conduction prolongs the VP, and thus reduced excitability becomes inherently proarrhythmic in a multicellular setting.
Potassium channel block influences membrane properties in a different manner. Reducing the magnitude of outward currents active during repolarization will prolong the APD. At the cellular level, extending the APD is antiarrhythmic, in that responses to any premature stimuli that fall within the APD will be suppressed. In a multicellular preparation, however, the wavelength will be prolonged, and if the wavelength is large than the core perimeter (negative excitable gap), then the core will become unstable as the wave front moves toward more excitable tissue. If the spatial domain is insufficient to support an unstable core, then the arrhythmia will be self-terminating. However, in the presence of structural disease, perhaps resulting in increased nonuniform anisotropy secondary to fibrosis, then the spatial requirements for reentry may be sufficiently reduced such that sustained arrhythmias can be supported.31 As discussed below, an action potential wavelength of 15 cm implies that the amount of tissue available to support reentry of a high-rate arrhythmia is borderline under normal conditions, indicating that disease resulting in structural defects that slow propagation may play a very important role in arrhythmogenesis.
Spiral reentry also
requires a critical mass of excited
tissue.5 32 33 In cardiac tissue, for
instance, only a
small spiral fragment may be able to develop. Consider a propagation
velocity of 50 cm/s and an APD of 0.3 seconds. The width of the spiral
wave (wavelength) is thus approximately 15 cm, a significant fraction
of the myocardial surface. For one revolution of the spiral wave front
to develop, a heart with a minimum circumference of  30 cm would be
required. Thus, the geometric extent of myocardial tissue significantly
constrains the development of a spiral wave, and only a small fragment
may be able to develop. Consequently, one can hypothesize that ECGs
displaying a polymorphic pattern may often be associated with
high-rate tachycardias that reveal a high conduction velocity
(long wavelength). As the conduction velocity is reduced, the
wavelength is reduced and a longer spiral is able to develop,
diminishing the influence of the tip on the ECG. This model provides a
conceptual basis for relating monomorphic ECGs with low-rate
tachycardias and polymorphic ECGs with high-rate
tachycardias.
30 cm would be
required. Thus, the geometric extent of myocardial tissue significantly
constrains the development of a spiral wave, and only a small fragment
may be able to develop. Consequently, one can hypothesize that ECGs
displaying a polymorphic pattern may often be associated with
high-rate tachycardias that reveal a high conduction velocity
(long wavelength). As the conduction velocity is reduced, the
wavelength is reduced and a longer spiral is able to develop,
diminishing the influence of the tip on the ECG. This model provides a
conceptual basis for relating monomorphic ECGs with low-rate
tachycardias and polymorphic ECGs with high-rate
tachycardias.
Do Polymorphic Reentrant Processes Require Dispersion of
Refractoriness?
Although we limited our investigations to spiral wave
reentry once
the spiral was initiated, we had to use some method to initiate the
spiral. Nonuniform cellular properties have often been cited as
important for initiating such reentrant processes.24
Clearly, refractory properties at the S2 stimulation site
must be nonuniform. Since we studied an array of identical cells, we
induced a transient nonuniform distribution of refractory states by
using different S1 and S2 stimulation sites.
The propagating S1 wave front thus provides the
nonuniformity. Since reentry readily can be initiated in an
homogeneous array, one might question whether dispersion of
refractory properties is necessary for polymorphic processes.
Recently, a model of torsade de pointes was proposed by Abildskov and Lux22 that was based on an array of cells with differing refractory properties. Their model resulted in a mobile point of reentry and produced a variety of polymorphic electrograms. The size of their array of excitable cells was small, such that only a small fragment of a wave front was able to develop. It was not clear, however, whether this nonuniformity was essential for their results.
Pertsov and colleagues12 observed polymorphic electrograms during spiral wave activation in a small slice of tissue in which a spiral fragment drifted along a gradient of excitability. In their numerical studies, they superimposed a gradient of excitability on their grid of excitable cells and found that polymorphic ECGs could be produced by the drifting spiral wave. To our knowledge, however, our results represent the first demonstration that spiral tip precession can produce a polymorphic ECG in an array of identical cells in which development of the wave front is constrained by the size of the medium. Our results, based on a simplified model of a cardiac cell (a single activation and a single recovery current), suggest that altering the balance between depolarizing and repolarizing currents amplifies spiral tip precession (which produces polymorphic ECGs) and is generic for an excitable medium, independent of the detailed nature of the depolarizing and repolarizing currents and cellular connectivity. From this foundation, it is feasible to explore the role of different medium properties (anisotropic connectivity, nonuniform cellular properties, multiple ionic currents) and assess whether these properties amplify or attenuate behavior exhibited by the underlying "generic" medium.
Relationship Between Polymorphic Processes and the Spiral
Core (Flowers)
Winfree25 first noted precession
(meandering) of the
spiral tip in studies of an excitable chemical reagent. For years,
spiral tip motion has been the subject of some study, but its
relationship to macroscopic events has remained unclear. Recently,
Winfree15 suggested that spiral tip precession might be
associated with torsade de pointes. Here we have demonstrated that when
development of a spiral wave front is constrained to only a small
fragment, then the region of the tip can dominate the ECG computed from
all cells in the region. As the size of the medium increases, the
polymorphic nature of the ECG diminishes.
The pattern of rotation of
the spiral wave reflects a compound process:
precession of the tip around an unexcited region with frequency
ft and rotation of the spiral about the tip with a
frequency fs. The ratio of these two frequencies determines
the configuration of the flower that is inscribed. More specifically,
if ft is an exact multiple of fs, then
p=1 + fs/ft, where p is the
number of petals. If the relationship between these two frequencies is
not an exact multiple, then each successive inscription of the flower
will be rotated (see Fig 5B![]() ) and the number of reentrant cycles
for
each polymorphic cycle will not be exact.
) and the number of reentrant cycles
for
each polymorphic cycle will not be exact.
This relation suggests the possibility of assessing myocardial properties by analyzing the fine structure of the polymorphic pattern. A low tip-precession frequency hypothetically implies a large, multipetaled flower that results from a prolonged repolarization process (long QT) and an unstable spiral core. A large flower may amplify the instability of the spiral wave front. For instance, the inscription of a large flower in real, inhomogeneous tissue may expose the wave front to obstacles that can act in either of two ways: (1) the wave front can collide with an obstacle and produce wave fronts that interfere with the incident wave front, resulting in termination of the arrhythmia, or (2) collision may result in the development of new spirals with fractionation of the reentrant process perhaps producing a fibrillatory rhythm leading to sudden cardiac death.19 With a small flower, if the spiral succeeds in making one revolution, then there is a high probability that the spiral will be able to rotate forever, leading to a sustained tachycardia and a monomorphic ECG.
Modulating Spiral Wave Behavior With Altered Membrane
Properties
Our results suggest that maneuvers that reduce repolarizing
currents are proarrhythmic in that they destabilize the unexcited core
by increasing the wavelength (APD · velocity) relative to the
perimeter of the core region. When the wavelength is prolonged beyond a
critical value, our results suggest that the region of block becomes
unstationary, producing a polymorphic ECG similar to that described
in clinical studies.10 An interesting clinical test of the
relation between wavelength and core size is found in Reference 10.
Horowitz et al10 found that in patients with inducible
polymorphic tachyarrhythmias, in the presence of
procainamide, only a monomorphic process could be induced. We
hypothesize that the sodium channel block by procainamide
reduced cellular excitability, thereby increasing the size of the
unexcited core and, in parallel, reduced the action potential
wavelength secondary to slowed conduction. The resultant monomorphic
ECG suggests that the perimeter of the stationary core was greater than
the wavelength.
Can cellular and multicellular models assist in evaluating drug effects? Although we have focused on reentry in the presence of drug-induced channel blockade, the evaluation of a drug should also include its effect on the initiation process. Consider sotalol and amiodarone. Both agents can block ß-receptors, can prolong the APD through potassium channel blockade, and have some affinity for the sodium channel. They differ, however, with respect to calcium channel blockade; only amiodarone exhibits significant affinity for calcium channels.34 35 On the basis of these data, we would expect that both would exhibit similar effects with regard to sustaining reentry and promoting polymorphic behavior. However, they would differ radically with respect to initiation of reentry via EADs. The ability of either agent to block ß-receptors will reduce the likelihood of EADs secondary to increased calcium current during intervals of high sympathetic activity. However, because of its additional calcium blocking ability, amiodarone34 can significantly reduce the probability of EADs by attenuating one source of inward current available at plateau potentials. Thus, our model suggests that amiodarone will exhibit fewer proarrhythmic effects than sotalol even if the QTc remains prolonged.
Quinidine is another agent used to control tachyarrhythmia that exhibits both significant sodium and potassium blocking properties.36 37 If torsade de pointes is associated with quinidine use, it often appears early during the course of medication. The pharmacokinetics of quinidine relative to each channel type is not well characterized, but consider the following hypothetical scheme. We assume a high affinity of quinidine for potassium channels and a lower affinity for sodium channels. On the basis of this hypothesis, we would expect early arrhythmias associated with quinidine use to be polymorphic (secondary to the dominance of potassium channel block and low plasma concentration of quinidine) and arrhythmias occurring later during the course of treatment (after the plasma level has increased to the sodium channel kD) to be monomorphic (secondary to sodium channel block). Of course, this oversimplifies the complex nature of drug-channel interactions, but this example illustrates how one can translate cellular electrophysiological results to a clinical setting and anticipate proarrhythmic possibilities.
In addition to drug modification of channel properties, hypokalemia also reduces the conductance of the inward rectifier potassium channel.17 Our numerical studies of the Beeler-Reuter21 model revealed that only a 10% reduction in gK was necessary to destabilize the spiral core. This suggests that not only can low concentrations of quinidine or other class III agents exhibit proarrhythmic properties but modest shifts in potassium concentration may also do so. A 10% reduction in gK can be achieved by approximately a 20% reduction in extracellular potassium concentration,17 eg, a reduction from 4 to 3.2 mmol/L.
In summary, our results suggest that, similar to the antiarrhythmic and proarrhythmic properties associated with modulating the complementary sodium current,1 2 3 the cellular antiarrhythmic properties associated with reduction in repolarizing currents are directly coupled with a multicellular proarrhythmic property: that of destabilizing the reentrant core and sustaining a polymorphic process. Further numerical and in vitro studies are required to confirm hypotheses suggested by our studies.
| Selected Abbreviations and Acronyms |
|---|
|
| Acknowledgments |
|---|
Received July 18, 1994; revision received December 21, 1994; accepted January 2, 1995.
| References |
|---|
|
|
|---|
This article has been cited by other articles:
 |
B.-R. Choi, F. Burton, and G. Salama Cytosolic Ca2+ triggers early afterdepolarizations and Torsade de Pointes in rabbit hearts with type 2 long QT syndrome J. Physiol., September 1, 2002; 543(2): 615 - 631. [Abstract] [Full Text] [PDF] |
||||
 |
P. Taggart, P. M.I. Sutton, M. R. Boyett, M. Lab, and H. Swanton Human Ventricular Action Potential Duration During Short and Long Cycles: Rapid Modulation by Ischemia Circulation, November 15, 1996; 94(10): 2526 - 2534. [Abstract] [Full Text] |
||||
 |
A. Patwardhan, S. Moghe, K. Wang, and F. Leonelli Frequency modulation within electrocardiograms during ventricular fibrillation Am J Physiol Heart Circ Physiol, August 1, 2000; 279(2): H825 - 835. [Abstract] [Full Text] [PDF] |
||||
 |
F. H. Samie, R. Mandapati, R. A. Gray, Y. Watanabe, C. Zuur, J. Beaumont, and J. Jalife A Mechanism of Transition From Ventricular Fibrillation to Tachycardia : Effect of Calcium Channel Blockade on the Dynamics of Rotating Waves Circ. Res., March 31, 2000; 86(6): 684 - 691. [Abstract] [Full Text] |
||||
 |
T. Ikeda, M. Yashima, T. Uchida, D. Hough, M. C. Fishbein, W. J. Mandel, P.-S. Chen, and H. S. Karagueuzian Attachment of Meandering Reentrant Wave Fronts to Anatomic Obstacles in the Atrium : Role of the Obstacle Size Circ. Res., November 19, 1997; 81(5): 753 - 764. [Abstract] [Full Text] |
||||
 |
T. Ikeda, L. Czer, A. Trento, C. Hwang, J. J. C. Ong, D. Hough, M. C. Fishbein, W. J. Mandel, H. S. Karagueuzian, and P.-S. Chen \E Induction of Meandering Functional Reentrant Wave Front in Isolated Human Atrial Tissues Circulation, November 4, 1997; 96(9): 3013 - 3020. [Abstract] [Full Text] |
||||
 |
C. A. Athill, T. Ikeda, Y.-H. Kim, T.-J. Wu, M. C. Fishbein, H. S. Karagueuzian, and P.-S. Chen Transmembrane Potential Properties at the Core of Functional Reentrant Wave Fronts in Isolated Canine Right Atria Circulation, October 13, 1998; 98(15): 1556 - 1567. [Abstract] [Full Text] [PDF] |
||||
 |
J. Beaumont, N. Davidenko, J. M. Davidenko, and J. Jalife Spiral Waves in Two-Dimensional Models of Ventricular Muscle: Formation of a Stationary Core Biophys. J., July 1, 1998; 75(1): 1 - 14. [Abstract] [Full Text] |
||||
 |
Y.-H. Kim, A. Garfinkel, T. Ikeda, T.-J. Wu, C. A. Athill, J. N. Weiss, H. S. Karagueuzian, and P.-S. Chen Spatiotemporal Complexity of Ventricular Fibrillation Revealed by Tissue Mass Reduction in Isolated Swine Right Ventricle . Further Evidence for the Quasiperiodic Route to Chaos Hypothesis J. Clin. Invest., November 15, 1997; 100(10): 2486 - 2500. [Abstract] [Full Text] |
||||
|
|
| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |
| CIRCULATION | ART, THRO, VASC BIO | ALL AHA JOURNALS |
| CIRCULATION RESEARCH | HYPERTENSION | STROKE |